I have been testing a new method that does double elution of DNA from a source deep well plate, using 50ul tip, 8 channel, Hamilton starlet.
There were no issues when transferring into 1.5mL screw cap tubes in the 32 tube carrier.
I first transferred 20-50ul of DNA into the empty tube, and then repeat that again (new tip) with another 20-50ul into the tubes which now have some liquid in them.
cLLD wasn’t working reliably for dispense and i was worried also about splashing, so I elute 1mm from the bottom.
I recently switched to elute into Hamilton storage 0.3mL 2D matrix barcoded tubes, and suddenly we are seeing a lot of contamination between wells.
We have confirmed contamination is happening at this step and now think it is due to static.
Specifically, we noticed sometimes there is liquid on the exterior of the tip (side not bottom) after the second transfer, and assume static pulls some liquid off causing the contamination.
Of note - lab humidity is 40-60%, contamination is worse with higher volumes (50ul vs 20ul), we dont see drips at the end of the tip, contamination has so far only been seen on the 2nd elution step (when dispensing into tubes with liquid), slowing dispense speed didn’t help.
Dispensing so the tip is above (not in) the liquid and/or waving an antistatic strip over the plate prior to use has helped reduce contamination.
Has anyone else experienced static causing contamination, and/or static issues with these or similar tubes, on the hamilton?
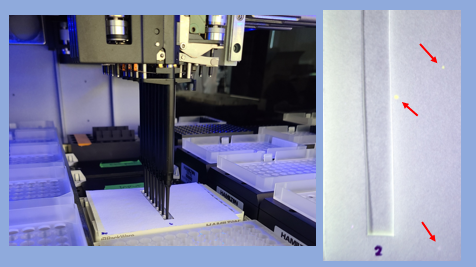
I attach a picture of the tubes, as well as an example of the contamination that we verified by adding a fluroescent dye to the reagent and placing paper ontop of the plate to “catch” the contaminanting droplets.
Note, that we’ve seen droplets spread multiple columns away from the source column.